Supplement Summary: O2 and Hyperbaric Oxygen Therapy
The Element: O2
Human life depends on oxygen, specifically O2. The air in Earth's atmosphere is made up of approximately 78 percent nitrogen and 21 percent oxygen. This ratio holds constant at all altitudes, but the density of the air decreases at higher altitudes and increases closer to sea level with increasing atmospheric pressure.
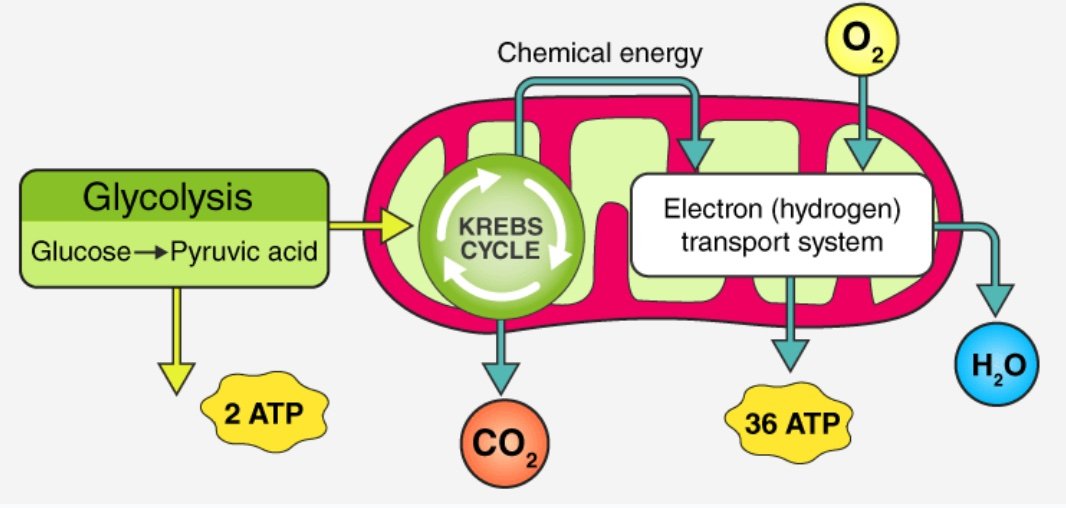
The main function of O2 in the body is to facilitate the production of cellular energy in the form of adenosine triphosphate or ATP. Our cells perform so-called aerobic respiration, which takes places in the mitochondria.
O2 acts as an final electron acceptor, which leads to ATP synthesis in a process known as oxidative phosphorylation. Carbon dioxide and water are created as byproducts.
The Therapeutic Use of Supplemental O2
If the amount of oxygen circulating in the blood decreases due to an inadequate amount in the air, the lungs’ inability to inhale and exhale appropriately, or the circulatory system not pumping blood efficiently, the body cannot operate normally and a condition called hypoxemia can develop. When hypoxemia worsens, and the oxygen levels in the tissues and organs begin to fall, hypoxia occurs. Normal oxygen saturation levels in the blood range from 95 percent to 100 percent, or 75 mmHg to 100 mmHg. When levels fall below 90 percent or 60 mmHg, hypoxemia occurs, and supplemental oxygen is required to increase the oxygenation of the blood.
Oxygen therapy helps people with lung diseases or breathing problems get the oxygen their bodies need to function. This oxygen is a supplement (i.e. additional) to the oxygen that is in the air. There are a number of different types of oxygen therapies that can be used including oxygen gas, liquid oxygen, oxygen concentrators, and hyperbaric oxygen therapy. Common ways to deliver oxygen in medical settings include nasal cannulas, face masks, positive pressure ventilation, or tracheostomy tubes for patients who are intubated.
Hyperbaric Oxygen Therapy (HBOT)
Hyperbaric oxygen therapy (which we will refer to as HBOT) is an extraordinary support in the handling of hypoxia and other hypoxia-related phenomena by increasing blood and tissue levels of oxygen. This involves a patient lying in a pressurized chamber filled with 100% pure oxygen for a specific amount of time to increase the amount of oxygen taken in with each breath.
Henry’s gas law states that the amount of dissolved gas in a liquid is directly proportional to the partial pressure of the gas above the liquid.
At a pressure greater than 1 atm absolute (ATA)--i.e. normal atmospheric pressure at sea level–the amount of oxygen delivered to the body’s tissue increases.
Most oxygen carried in the blood is bound to hemoglobin, which is ~97% saturated at atmospheric pressure. Some oxygen is carried in the plasma, and this portion is increased at pressure due to Henry's Law. Effectively, the blood can be hyperoxygenated by dissolving oxygen within the plasma, which can then reach physically obstructed areas where red blood cells cannot pass. This hyperoxemia in the blood can then create states of hyperoxia in the tissues (or relative normoxia in tissues with ischemia) even without the contribution from hemoglobin.
HBOT vs. mild (m) HBOT
Mild hyperbaric oxygen therapy (mHBOT) is a type of therapy that involves breathing in pure oxygen while in a pressurized chamber with a slightly higher air pressure than normal atmospheric pressure. mHBOT is delivered via a portable hyperbaric chamber, made of flexible materials, such as reinforced fabric or PVC, which can be pressurized to 1.5-1.7 atmospheres absolute (ATA). This therapy is called "mild" because the pressure used is lower than that used in traditional or “medical grade” HBOT. However, any pressure above 1.0 ATA will allow extra oxygen to enter into the body and pressures between 1.0 ATA and 1.7 ATA are still considered “hyperbaric”. Currently, mHBOT is only approved by the FDA for the treatment of altitude illness. However, as the number of these chambers has increased, as they are being used more commonly in off-label indications.
It is important to note that much of the research examining mHBOT is still preliminary, and clinical research is needed to fully understand its efficacy. Some of the potential benefits of mHBOT based on preliminary data include:
Improved oxygen supply: mHBOT can increase the amount of oxygen that is available to the body's tissues, which may help to improve healing and recovery.
Reduced inflammation: mHBOT has been shown to reduce inflammation in some cases, which may help to alleviate pain and improve overall health.
Improved immune function: mHBOT has been shown to stimulate the immune system, which may help to fight off infections and other illnesses.
Enhanced wound healing: mHBOT may help to improve the healing of wounds, particularly in cases where there is poor circulation or a lack of oxygen supply to the affected area.
Reduced symptoms of certain health conditions: mHBOT has been studied as a potential treatment for a range of conditions, including chronic fatigue syndrome, fibromyalgia, traumatic brain injury, and some neurological disorders.
Biomolecular and cellular mechanisms of action
It seems that one of the most important ways by which this extra oxygen exerts its biomolecular and cellular effects is via what is known as the “hyperoxic hypoxic paradox.”
Low levels of oxygen, or hypoxia, is one of the most powerful inducers of gene expression, metabolic changes, and regenerative processes, including angiogenesis (growth of new blood vessels) and stimulation of stem cell proliferation, migration, and differentiation. Our cells have specialized chemoreceptors and metabolic switches to regulate the response. However, this response is triggered by the relative level of oxygen, meaning fluctuations in the free oxygen concentration rather than the absolute level of oxygen can be interpreted at the cellular level as a lack of oxygen. Thus, the normoxia followed by repeated intermittent hyperoxia is interpreted by the body as relative hypoxia and induces many of the mediators and cellular mechanisms that are usually induced during true hypoxia.
Most evidence so far points to the mitochondria as the main sensing organelles which signal the onset of hypoxia by generating reactive oxygen species (ROS) signals by the electron transport chain. When ROS are released to the intermembrane space, they interplay with the activation of enzymes, transcription factors, and post-translation responses. As Shai Efrati and colleagues have demonstrated, the use of intermittent hyperoxia via HBOT can stimulate tissue regeneration without the hazardous effects of hypoxia.
Acute Physiological Effects
The hyperoxic hypoxic phenomenon created by HBOT induces a cellular cascade.
The transcription factor hypoxia-inducible factor- 1 (HIF-1), when stabilized by hypoxic conditions, regulates over 100 genes essential for survival in oxygen-deprived conditions including glycolysis enzymes, enzymes decreasing the basal respiratory rate, and the vascular endothelial growth factor (VEGF) to induce angiogenesis which improves tissue perfusion.
HIF-1 induces VEGF-A production which in turn activates vascular cells to initiate angiogenesis and arteriogenesis, vasodilatation activity, and microvascular permeability. HIF-1 also interacts with Sirtuin 1 (SIRT1), which is involved in various mechanisms regulating apoptosis, inflammation, and senescence, which are associated with aging-related diseases. The decreased level of SIRT1 during aging is considered to be a major metabolic pathway that reduces the number and size of mitochondria and causes aging-related diseases. An energetic stress, such as transient relative hypoxia, stimulates the enzymes that trigger mitochondrial biogenesis, the process by which new mitochondria are formed by growth and division of preexisting mitochondria. Mitochondrial biogenesis is crucial for preserving human cell integrity. And finally, HIF-1 activity due to transient hypoxia can induce the proliferation, migration, and differentiation capacity of stem cells.
Source: Hadanny A, Efrati S. The Hyperoxic-Hypoxic Paradox. Biomolecules. 2020.
It is plausible and increasingly apparent that the hyperoxic-hypoxic paradox underlies the biomolecular and cellular effects of HBOT. The stimulus of oxygen fluctuations from hyperoxia to normoxia generates a hypoxia-mimicking state that triggers adaptive cellular cascades without some of the hazardous effects of hypoxia.
The cells interpret the change from hyperoxia back to normoxia as relative hypoxia which induces HIF-1 expression and, with regular and repeated exposure, decreases the ratio of reactive oxygen species to antioxidant scavengers. Evidence from preclinical as well as from clinical studies demonstrating that repeated HBOT sessions induce the crucial elements for angiogenesis, HIF-1 mediated VEGF expression, and endothelial progenitor cells. Intermittent hyperoxic exposure increases the activity of Sirtuin 1, which along with other sirtuins, appears to be an integral part of an important cellular defense mechanism against oxidative stress and an activator of mitochondrial biogenesis. And growing data from preclinical and clinical studies demonstrate the cumulative effect of repeated intermittent hyperoxia by HBOT on proliferation and mobilization of various subtypes of stem cells.
Source: Schottlender N, Gottfried I, Ashery U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules. 2021
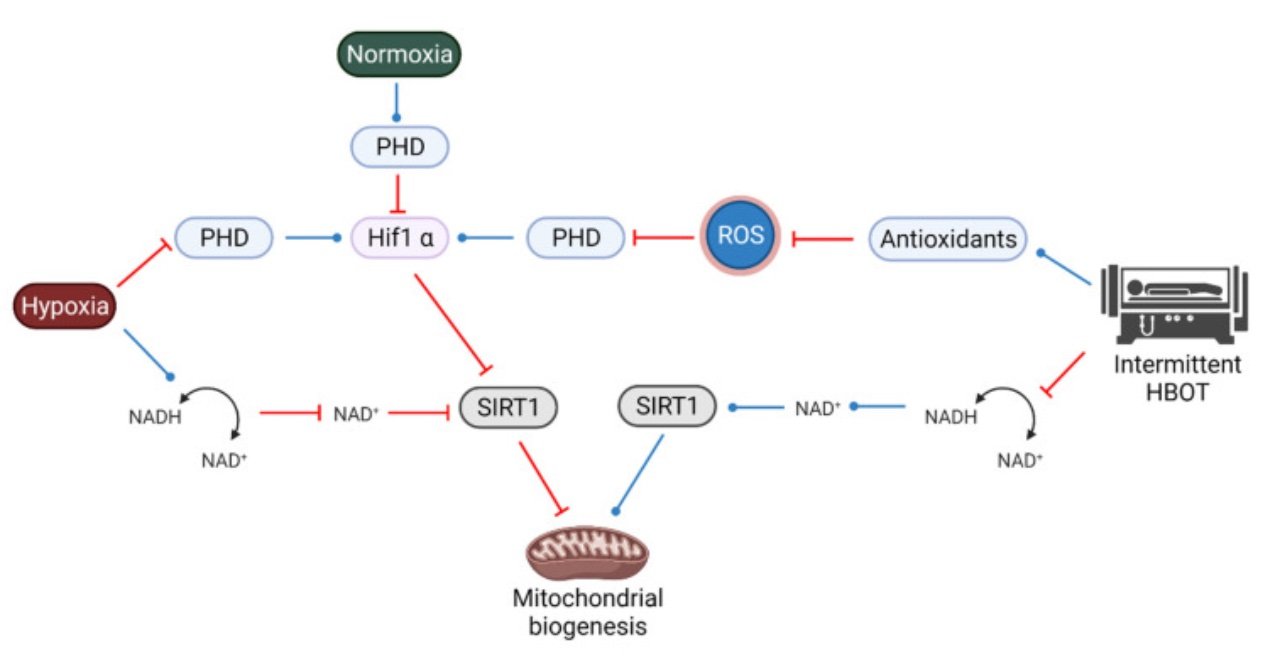
The complicated interplay between SIRT1, HIF1a and ROS. Intermittent HBOT elevates antioxidants, which leads to inhibition of PHD, therefore mimicking a hypoxic state and also activating HIF1a. Intermittent HBOT also activates SIRT1, leading to an activation of mitochondrial biogenesis.
Long Term Physiological Effects
Adaptive and durable physiological effects of HBOT manifest over time with repeated exposures– on the order of 20 to 60 sessions. These effects include new tissue formation via stem cells and angiogenesis, suppression of cellular senescence, reduced inflammation, and increased antioxidant activity.
Source: Fu Q, Duan R, Sun Y, Li Q. Hyperbaric oxygen therapy for healthy aging: From mechanisms to therapeutics. Redox Biol. 2022.
HBOT, at higher pressures of >2ATM, is a well known and widely utilized modality in hospitals and wound care centers for skin collagen & tissue regeneration in the setting of wounds, infections, and burns. HBOT effectively activates fibroblasts and mobilizes stem cells–the key building blocks for new cells and tissue.
In order for this new tissue to grow and thrive, it needs a continuous supply of vital nutrients, which are delivered by the bloodstream. HBOT has been demonstrated to increase angiogenesis, or the creation of new blood vessels. HBOT stimulates the body systemically to produce new capillaries and blood vessels. This is particularly important for injured or aging tissue because the addition of hyperbaric oxygen therapy can support the body’s ability to grow new blood vessels from these areas where circulation has been compromised and limited. This is an exciting added benefit that has been demonstrated from HBOT because the blood vessels are produced basically ‘from scratch’ (‘de novo’ in medical terms). This is through HBOT’s ability to stimulate the bone marrow to produce new stem cells (as previously discussed) and in this case they are blood vessel cells.
In areas of the body that are damaged, infected, or experiencing chronic stress, circulation might be diminished or blocked due to inflammation. Studies have found that HBOT can help decrease the inflammatory response by reducing the release of inflammatory cytokines, substances like Interleukin-1 (IL-1), Tumor Necrosis Factor-alpha (TNF-α), and Interleukin-6 (IL-6). HBOT can enhance the activity of white blood cells, which can help to reduce inflammation and fight infection. And finally, HBOT has been shown to reduce edema, the swelling caused by excess fluid trapped in inflamed areas of the body.
At the cellular level, two key hallmarks of the aging process include telomere length shortening and cellular senescence. When telomeres–the end caps that maintain the integrity of our DNA arranged in chromosomes– shorten to a critical length, cells cannot replicate and progress to senescence or programmed cell death. In a preliminary study that evaluated the telomere length and senescence in the blood cells of thirty-five healthy independently living adults (aged 64 and older) who received 60 daily HBOT exposures, telomeres length increased by over 20% and the number of senescent cells decreased significantly. Further studies and evidence are underway to investigate the senolytic effects of HBOT.
Last but not least, HBOT can elevate antioxidant activity. Although oxygen is needed for ATP production, it can sometimes have deleterious effects when it interacts with other molecules, exchanges electrons and is transformed into reactive oxygen species (ROS). These ROS have what are known as free radicals that can damage tissues by “stealing” electrons from lipids, proteins, DNA, etc., rendering them inactive or reactive themselves. These free radicals are cleared by enzymatic (e.g. superoxide dismutase, catalase, heme oxygenase 1, thioredoxin and glutathione-dependent peroxidase and reductase) and nonenzymatic/endogenous (e.g. vitamin C, vitamin E, glutathione, melatonin, uric acid and β-carotene) antioxidants. Ultimately, the balance between free radical levels and antioxidant levels and activity will determine the extent of the oxidative stress.
Given the excess oxygen delivered by HBOT, it elevates ROS production via the mitochondria. Importantly, these ROS (as well as reactive nitrogen species) serve as signaling molecules in transduction cascades that support healing, cell survival and proliferation through the activation of aforementioned factors like HIF1α, SIRT1, VEGF and other growth factors and hormones. However, repeated HBOT, when provided at the appropriate therapeutic and approved limits, also induced a concurrent increase in antioxidant levels and activity that help the mitochondria function without disturbing the redox balance and even enhancing their activity.
Health Impacts of Hyperbaric Oxygen Therapy
Over the past few decades, HBOT has been widely promoted for many ‘off-label’ conditions, causing much controversy in the medical field, from both skeptics to proponents. HBOT has been approved in 14 medical indications by the Undersea and Hyperbaric Medical Society - particularly for crush injuries, abscess, necrotizing infections, radiation injury, and burns. However, these therapies are typically completed in so-called “hard chambers” at >2atm, and under medical supervision. And much of the research on the health impacts of HBOT have been investigated in hard, medical grade chambers. Below we discuss the impacts of HBOT across different chambers, pressures, and protocols before a brief discussion comparing HBOT to mild HBOT.
Pain reduction (Fibromyalgia)
Several studies in humans utilizing HBOT have demonstrated relief in pain. It is thought that the anti-inflammatory effects are likely the key mechanism for this therapeutic effect. A population that has been highly studied for pain relief have been those diagnosed with fibromyalgia. HBOT appears to exert direct effects on brain activity, chronic pain and immune dysregulation, therefore improving quality of life of patients with fibromyalgia. Specifically, HBOT effects on reducing proinflammatory cytokines production by CD4 T cells was noted in this population. In a recent systematic review and meta-analysis HBOT was shown to have a positive effect in improving pain, tender points, fatigue, multidimensional function, patient global and sleep disturbance in FM, with reversible side effects and, interestingly, low pressure (less than 2.0 atmospheric absolute) specifically appeared to be beneficial to reduce adverse events.
Brain Health
In recent years, researchers have begun to refocus on the cognitive protective effects of HBOT in the context of normal aging. These cognitive effects on brain health have been noted, in both healthy older adults and individuals with age-related Alzheimer's and vascular dementia.
In a randomized controlled trial involving 63 healthy aging subjects, HBOT, utilized in a repeated 60 daily sessions protocol, was shown to induce cognitive enhancements in clinical aspects including attention, information processing speed and executive functions, likely by an increase in regional cerebral blood flow. It's worth noting that the study excluded transient effects of oxygen, as all changes were evaluated at 1–2 weeks after the last session. The increase in CBF was later confirmed by another study of HBOT in elderly individuals suffering from significant memory loss.
Interestingly, many sufferers from mold toxicity complain of cognitive deficits including: memory problems, inability to focus (brain fog), and slow reaction times. In addition to the data in older adults, 10 sessions of hyperbaric oxygen therapy has been shown in one study to cause improvements in attention span and reaction time in these patients. Moreover, this study revealed that these benefits were seen at only 1.3 ATA (lower pressure) and while using oxygen connections closer to room air than the conventionally-used 100% oxygen delivery systems.
Behavioral Health
It has been hypothesized that hyperbaric oxygen therapy may be beneficial in the treatment of certain mental health disorders such as post-traumatic stress disorder occurring in the setting of a traumatic brain injury. The proposed mechanism of action of hyperbaric oxygen in traumatic brain injury is multifold, and begins by increasing oxygenation of blood and tissues to supraphysiological levels which results in the improvement of neuronal functioning by the reactivation of metabolic or electrical pathways. Secondarily, stem cell mobilization to sites of injury, immune modulation and impact on neurotransmitters have also been hypothesized as possible mechanisms.
Cumulative evidence from post-stroke and traumatic brain injury studies also demonstrate that HBOT induces neuroplasticity in the chronic metabolic dysfunctional brain regions even years after the brain insult. Specifically, the potential beneficial effects of HBOT on PTSD were investigated in combat veterans with TBI which is commonly combined with PTSD. In most of the randomized controlled studies, a significant clinical improvement in PTSD symptoms was demonstrated including improving brain microstructure and functionality in veterans with treatment resistant PTSD.
There may also be indications for oxygen therapy in diagnoses of depression. One study has examined different percentages of inspired oxygen (21% or 35%) at normobaric conditions overnight for 4 weeks. Some, though not all measurements of depression demonstrated improvement after 35% inspired oxygen concentration compared to 21%.
With regard to hyperbaric oxygen therapy, a recent meta-analysis of 27 clinical trials (17 randomized) by Liang et al. it was found that the use of HBOT is effective both as add-on treatment as well as in monotherapy and significantly reduces the severity of post-stroke depression on the HAMD-17 scale. Further, one pilot study showed that HBOT may be effective in reducing depression and anxiety in patients with spinal cord injury, but further research is needed to confirm its effectiveness overall and in other populations.
Skin Health
Oxygen has been widely known to improve a wide variety of skin health issues, including necrotizing infections, skin grafts, and wound management. However, a recent prospective clinical trial demonstrated that repeated intermittent hyperbaric exposures also had dramatic aging-modulating effects on the skin, illustrated as decreased senescent cells, increased elastic fiber length and stability and collagen density, and induced angiogenesis. The therapy included 60 daily sessions, five sessions per week within a three-month period. Each session included breathing 100% oxygen by mask at 2ATA for 90 minutes with five-minute air breaks every 20 minutes. However, in another study in healthy individuals, it was reported that hyperbaric exposure at 1.25 ATA with 32% oxygen led to both the fading in melanin pigmentation induced by UVB irradiation and the decrease in senile spot size, indicating potential use in treatment of skin photoaging.
Metabolic Health in Healthy Aging and Type 2 Diabetes
Glucose homeostasis depends on appropriate insulin secretion and the sensitivity of receptors to insulin, both of which are impaired with advancing age. While no clinical trials to date have specifically looked at the effects of HBOT on glucose metabolism in healthy aging population, in secondary analysis of existing clinical trials involving healthy individuals of a wide range of ages, HBOT has shown a tendency to lower serum insulin, increase peripheral insulin sensitivity and reduce HbA1C. Thus, HBOT may benefit the aging populations in terms of glucose metabolism by attenuating age-related insulin resistance. In individuals with compromised metabolic health, as with type 2 diabetes, HBOT has shown clinical efficacy in lowering blood glucose and improving insulin sensitivity.
Empirical data also suggests that HBOT can induce penile angiogenesis and improve erectile function in men suffering from erectile dysfunction, particularly in the presence of diabetes or recovery after urethral reconstruction.
Cancer
The use of HBOT as part of the cancer therapy is not currently an approved indication, however accumulating evidence supports the role of HBOT in the inhibition of tumor growth and therapy success, by three main mechanisms: (1) By limiting cancer-associated hypoxia, (2) through the generation of ROS and RNS and (3) restoring immune function. Actual investigations show the promising role of HBOT in a wide variety of malignancies, including breast cancer, prostate cancer, head and neck cancer, colorectal cancer, leukemia, brain tumors, cervical cancer and bladder cancer.
COVID 19
While emerging, and somewhat controversial, it has been demonstrated that HBOT may help to inhibit SARS-CoV-2 replication in cell models by increasing the production of nitric oxide (NO) and reactive-oxygen and reactive-nitrogen species. Although it is still being evaluated scientifically, hypotheses are arising for COVID-19 treatment, with some preliminary studies finding an attenuation of the innate immune system, and increasing hypoxia tolerance in humans. HBOT may also offer potential support in the relieving of cytokine storm. Another preliminary study showed rapid alleviation of hypoxemia from the beginning of the treatment in patients with COVID-19 pneumonia. It should be noted, however, that COVID-19 is not yet an accepted indication for HBOT.
Performance Effects of Hyperbaric Oxygen Therapy
In recent years, researchers have demonstrated that mild hyperbaric oxygen under 1.25 ATA with 36% oxygen, can be effective against degenerative changes in the musculoskeletal system overall, although much of this data is in cell and animal studies. In rats, mild hyperbaric oxygen can prevent bone and muscle loss induced by unloading in while HBOT up to 2.5ATA accelerates satellite cell proliferation and myofiber maturation in injured muscle, suggesting that HBOT treatment accelerates healing and functional recovery. The specific mechanisms by which HBOT may improve muscle recovery is by acute increases in NO3-, VEGF, and bFGF levels, stabilizing HIF1α, and stimulation of collagen production by fibroblasts which encourage longer term blood vessel formation. Some animal studies have demonstrated improvements in ligamentous injuries with HBOT therapies in a variety of animal models, but data in humans is lacking, with only two older studies demonstrating inconclusive results.
In humans, one study examined the effect of HBOT on inflammation, the oxidative/antioxidant balance and muscle damage after exercise. Eighteen young men were asked to run for 60 minutes at 75-80% max heart rate in normobaric/normoxic or hypobaric/hypoxic environments. The HBOT treatments consisted of breathing 100% oxygen at 2.5 atmosphere absolute (ATA) for 60 min after exercise. Serum IL-6 and lactate dehydrogenase levels were improved in all conditions after HBOT and serum creatine kinase levels were improved after exercise in the hypobaric/hypoxic environment. These results suggest that acute exercise in both the normobaric/normoxic and hypobaric/hypoxic environments could induce temporary inflammatory responses and muscle damage, whereas HBOT treatment may be effective in alleviating exercise-induced inflammatory responses and muscle damage. However, this study did not investigate subsequent performance outcomes based on these observations.
Hyperbaric oxygen therapy has also become popular among injured athletes because of its purported benefits of tissue regeneration and accelerated recovery. However, while some studies have demonstrated benefit for subjective reports of delayed onset muscle soreness (DOMS), this finding has not been repeated consistently. Staples et al. demonstrated the effects of HBOT on the faster recovery of DOMS in athletes for the first time. Their protocol included hyperbaric exposures of 100% oxygen for 1 hour per day at 2.0 atm for up to 96 hours after exercise. However, several subsequent studies have shown inconsistent results and the most recent systematic review suggests that HBOT is ineffective for DOMS (see below). However, human studies are scarce despite widespread use of HBOT among athletes and require rigorous scientific studies before concluding if HBOT can facilitate the return to play of athletes.
Due to the ability of HBOT to potentially enhance aerobic activity by inducing mitochondrial biogenesis in skeletal muscle, it has also been proposed as a pre-conditioning method to enhance exercise performance. Villela and colleagues recently conducted a study where seventeen subjects were enrolled (10 men, 7 women, ages 26.5±1.3 years) and each completed 6 HIIT sessions over 2 weeks. Participants were randomized to breathing normobaric air (PiO2 = 0.21 ATM) or HBO2 (PiO2 = 1.4 ATM) during training. Vastus Lateralis (VL) muscle biopsies were performed before and after HIIT in both groups and V̇O2peak tests were conducted before and after training in a hypobaric chamber at PiO2 = 0.12 ATM. While HIIT significantly increased V̇O2peak in both groups, HBOT did not significantly affect outcomes.
The Dose Makes the Poison: Oxygen Toxicity
As is true for heat, cold, and any other natural element, the dose determines whether it is an adaptive stimulus or a noxious poison. Breathing oxygen at higher than normal partial pressure leads to hyperoxia and can cause oxygen toxicity or oxygen poisoning.
The clinical setting in which oxygen toxicity occurs is predominantly divided into two groups– one in which the patient is exposed to very high concentrations of oxygen for a short duration, and the second where the patient is exposed to lower concentrations of oxygen but for a longer duration. These two cases can result in acute and chronic oxygen toxicity, respectively.
Oxygen-derived free radicals have been proposed as being the probable etiological cause in the development of oxygen toxicity. When produced in excess, free radicals and oxidants generate a phenomenon called oxidative stress, a deleterious process that can seriously alter the cell membranes and other structures such as proteins, lipids, lipoproteins, and DNA. Prolonged exposure to elevated Po2, whether increased concentrations of oxygen inspired at atmospheric pressure or low concentrations of inspired oxygen at high ambient pressures, places humans at risk for pulmonary oxygen toxicity. The phenomenon of pulmonary toxicity, known as the Smith effect, is defined by pleuritic chest pain, substernal heaviness, coughing, and dyspnea secondary to tracheobronchitis and absorptive atelectasis which can lead to pulmonary edema. The risk of CNS O2 toxicity, known as the Bert effect, is a function of both Po2 and exposure time, directly proportional to both. CNS complications secondary to oxygen toxicity primarily include tonic-clonic convulsions and amnesia.
Other Risks and Safety Considerations
Because of the increased pressure and increased concentration of the oxygen during HBOT, potential risks include:
Ear and sinus pain
Middle ear injuries, including tympanic membrane rupture
Temporary vision changes
Lung collapse (rare)
Oxygen toxicity (as discussed above) both central nervous system and pulmonary toxicity depend upon the partial pressure of oxygen and the duration of exposure. Most HBOT protocols used today include repeated daily sessions limited to 60–90 min with oxygen partial pressure not exceeding 2.4 ATA, as well as air brakes every 20–30 min. Using those new protocols, HBOT is considered to be safe, while both pulmonary and oxygen toxicity are very rare.
Clinical Protocols
The protocols for the approved indications of HBOT are more established. Less so for off label use for the aforementioned health conditions and general wellness purposes. What is clear, however, is that consistent and long-term use is needed for HBOT to be an effective therapy that causes durable physiologic changes.
Generally, HBOT is limited to 60 – 90 minutes per session. A very common protocol that has been shown to give these physiological benefits is called the ’40 hour hyperbaric protocol’. This protocol has been varied in pressure and in time and is typically delivered over 5 consecutive days with a 2 day rest period. With this protocol, a full 40 hours of HBOT can be accomplished in one month (with twice daily applications) or two months (if only one HBOT session is performed daily).
One specific emerging protocol, which utilizes repeated hyperbaric exposures of 100% oxygen at 2 ATA for 90 min with intermittent air breaks to exaggerate and capitalize on the effect of the hyperoxic-hypoxic paradox.
Despite its great potential demonstrated in preclinical studies, mHBOT still lacks supporting data from clinical trials. We need to determine not only the efficacy and sustainability of existing protocols, but also the dose-response curves related to oxygen pressure, exposure time, frequency of intervals and number of sessions in order to optimize treatment conditions.