The Science and Scoop Behind Photobiomodulation (AKA “Red Light”) Therapy
An Overview of Photomedicine
Humans are biological systems. As biological systems, we are designed to absorb light. This can be observed clearly in our sleep–wake cycles, circadian rhythms, and in the production of vitamins. The light sensitive cells in our retinas called photoreceptors absorb photons of light in order for our brain to process and reflect a visual image. The suprachiasmatic nucleus, a specialized group of neurons in the hypothalamus, gets its cues from sunlight to regulate most circadian rhythms in the body. And the ultraviolet radiation from the sun converts 7-dehydrocholesterol to previtamin D3. Previtamin D3 is then thermally converted to vitamin D3 in the skin, and the vitamin is then transported to the liver to be activated for use in the body.
By the same token, too much light can have adverse effects on our biological system. The combination of too much sun and too little melanin can cause painful sunburns. Over a period of years repeated exposure to the sun can cause a wrinkly and leathery appearance to the skin known as photoaging. Scientific studies have shown that repeated UV exposure breaks down collagen and impairs the synthesis of new collagen needed for the skin to repair itself. And of course, excess UV light can induce skin cancer (both the dangerous malignant melanoma and the less dangerous non-melanoma skin cancer). Meanwhile, not getting enough UV exposure can contribute to seasonal affective disorder or even rickets (due to lack of Vitamin D).
It is clear that exposure to light has many important implications on human biology and behavior. Light—technically defined as the non-ionizing electromagnetic radiation between the wavelengths of 200 nm to about 10000 nm–is composed of packets of energy called photons. Light energy is capable of causing heating, mechanical effects and chemical reactions. The transfer of light energy through photon absorption can lead to many different consequences. Photomedicine includes both the study and treatment of diseases caused by exposure to light as well as the diagnostic and therapeutic applications of light for detecting and curing disease, also known as phototherapy.
Phototherapy involves the transformation of light energy to chemical, kinetic or heat energy in order to achieve a desired physiological result. Therefore, light that is used for therapeutic applications must be absorbed by a specific chromophore–a biological molecule that serves to capture or detect light energy–in the biological tissue.
A Brief History and Technical Terminology
The term photobiomodulation (PBM) describes the scientific mechanism whereby light influences biological processes. Photobiomodulation therapy (PBMT) describes the intentional application of non-ionizing forms of light sources including LASERS, LEDs, and broadband light, in the visible and near infrared spectrum for therapeutic purposes.
Until recently, PBMT often went by the name low level laser therapy (LLLT) or laser biostimulation based on its original discovery. In 1967 at the Semmelweis Medical University in Hungary, Endre Mester was trying to cure tumors with his low-power laser beam. Although the laser did not treat the tumors, he did observe a heightened rate of hair growth and accelerated wound healing in the rats in which he had surgically implanted tumors. This was the first indication that low-level laser light (rather than high power thermal lasers) could have its own beneficial applications in medicine.
LLLT was initially primarily studied for stimulation of wound healing, and reduction of pain and inflammation in various orthopedic conditions such as tendonitis, neck pain, and carpal tunnel syndrome.
In the 1990s, the National Aeronautics and Space Administration developed light-emitting diodes (LEDs) with near-monochromatic light at the red (670, 720 nm) and near-infrared (880 nm) end of the visible spectrum. Initially designed for plant growth experiments in space, these LEDs were also found to enhance cellular proliferation in vitro and to improve wound healing in a number of experimental and clinical studies.
The advent of light emitting diodes (LED) led to LLLT being renamed as “low level light therapy”, as it became more accepted that the use of coherent lasers was not absolutely necessary. However, the terminology remained fraught due to uncertainties in the exact meaning of “low level.” Based on recent consensus in the field, PBM and PBMT are now considered the terms of choice.
PBM Mechanism(s) of Action
PBM most commonly uses visible red light and/or near-infrared light (NIR) wavelengths in PBM (600–1100 nm). Red light has the largest wavelength of all colors within the visible spectrum (625–740nm), and near-infrared light, although outside of the visible spectrum, contains even larger wavelengths (750–1000 nm). These large wavelengths allow for very high tissue penetration of light. Because of this property, the duration of light exposure for red and infrared light treatments is short and only requires seconds to minutes to cause photobiomodulation.
The light source is placed near or in contact with the skin, allowing the light energy (photons) to penetrate tissue where it interacts with endogenous chromophores located in cells resulting in photophysical and photochemical changes that lead to alterations at the molecular, cellular and tissue levels of the body.
The mechanistic model and much of the preclinical evidence describes how light induces a complex chain of physiological reactions to impact mitochondrial respiration, gene expression, cell signaling, growth factor synthesis, and inflammatory modulation. Increasing progress has been made in understanding the mechanisms of action at a molecular, cellular and tissue-based level. This has generated an exponential increase in interest in PBM among both medical professionals and academic researchers.
Acute (Immediate) Effects
Perhaps most notably and well described, PBM stimulates photoreceptors within the cell’s mitochondria to increase synthesis of ATP–our primary energy currency. The work of Tiina Karu in Russia has been instrumental in identifying cytochrome c oxidase (unit IV in the respiratory chain) as a primary chromophore. These absorption peaks are mainly in the red (600–700 nm) and near-infrared (760–940 nm) spectral regions. The excitation of cytochrome c oxidase by light irradiation at this ideal wavelength is thought to enhance electron transfer and thus ATP synthesis. At the level of the cell, The mitochondrial membrane potential (MMP) is increased by PBM, leading to increased electron transport. The effect is more ATP, more ENERGY!
The immediate effect of PBM increases blood flow is thought to be due to an increase in the bioavailability of nitric oxide. The leading theory for the mechanism of action describes the inhibition of cytochrome c oxidase enzyme activity by nitric oxide, especially in injured or damaged cells. This inhibitory nitric oxide can be dissociated by photons of light that are absorbed by cytochrome C oxidase. Once released by photodissociation, nitric oxide acts as a vasodilator as well as a dilator of lymphatic flow. There is also evidence from in vitro studies showing that the gene that produces nitric oxide is upregulated by low energy laser irradiation. And finally, PBM can increase NO bioavailability by increasing its synthesis and helping to release it from intracellular stores.
Source: Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol. 2018 Mar;94(2):199-212.
Experimental evidence suggests that the relationship between PBM and oxidative stress may depend upon the preexisting stressors on the cell. This has been confirmed by in vitro experiments by Hamblin and colleagues who demonstrated that PBM increased reactive oxygen species (ROS) in physiologically resting neurons but protected oxidatively stressed neurons from cell death by reducing the levels of reactive oxygen species. It seems that while PBM induces a modest and dose-dependent increase in ROS production in normal cell lines, it appears to reduce ROS levels in cells previously exposed to oxidative stress. It is theorized that the spike of oxidation from light promotes an antioxidant effect and upregulation of glutathione reductase and superoxide dismutase. The vasodilatory effect combined with up-regulation of antioxidant defenses and reduced oxidative stress likely underlies the reduction in inflammation that is consistently observed with PBM.
Longer Term Effects
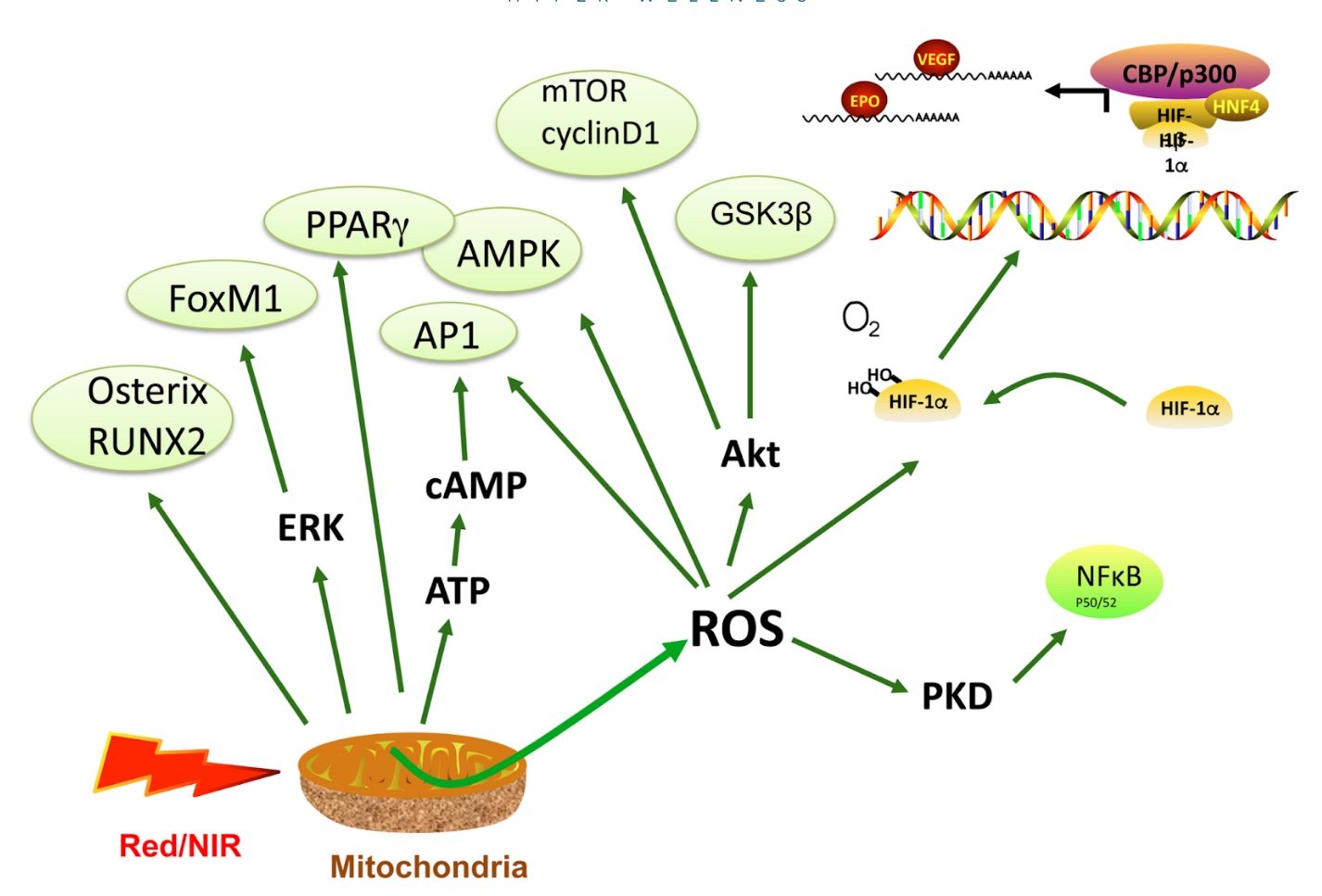
Many of the secondary mediators of PBM (reactive oxygen species, nitric oxide, cAMP) are able to activate transcription factors and signaling pathways. Activation of a vast array of transcription factors, as summarized in the visual below, is proposed to explain why a relatively brief exposure to light can have long-lasting results.
Source: Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol. 2018 Mar;94(2):199-212. This comprehensive review article cites all of the in vitro studies that show the mechanistic link between PBM and these transcription factors.
At a biochemical and cellular level, activation of these various signaling pathways lead to increased cell proliferation and migration, modulation in the levels of cytokines, growth factors and inflammatory mediators, and increased tissue oxygenation. The result of this restored cellular function on a tissue or system level include such benefits as accelerated wound healing and tissue regeneration, increased circulation, reduced acute inflammation, and reduced acute and chronic pain.
Source: http://photobiology.info/Photomed.html
Status of the Existing Scientific Evidence
The potential applications of PBMT are numerous and are being explored experimentally at the basic science, pre-clinical and clinical level. A majority of the research to date has been done using in vitro studies and animal models. On top of this imposing pile of pre-clinical evidence, there are some human studies, many of which are uncontrolled case reports. The clinical trials that do exist are very small with many variations in study design, particularly a wide variety of different kinds of light sources and treatment protocols including, illumination parameters (such as: wavelength, fluence, power density, pulse structure, etc.) and the fact that there is no agreement on the treatment schedule.
Unfortunately, these variations in study designs have led to an increased number of negative trials that were published and created some controversy, despite many positive pre-clinical results. Methodologic flaws, small patient cohorts, and industry funding compromises the quality of evidence. The clinical evidence is simply too disparate to generate any meaningful meta-analysis. And it remains unclear if light-emitting diode sources induce physiologic effects of comparable nature and magnitude to those of the laser-based systems used in most of the higher-quality studies.
Currently, the ubiquity and commercial success of PBMT has outpaced empirical approaches on which solid clinical evidence is established. Thus, the challenge is to prove its therapeutic utility in retrospect.
We must be clear that mechanistic plausibility does not constitute clinical evidence. However, it is crucial that we understand the mechanistic basis for PBM, so that we can engage in an honest dialogue with clients, and design better clinical studies to put claims of efficacy to the test.
Here is what we know to date about the therapeutic applications of PBM based on the existing clinical trials in human subjects.
Therapeutic Applications: Health Effects of PBMT
Pain
Clinical data show that the cutaneous applications of red and near-infrared lights has efficacy for the amelioration of pain associated with fibromyalgia, tendinopathy, burning mouth syndrome, trigeminal neuralgia, rheumatoid arthritis, osteoarthritis, and myofascial pain syndrome.
Skin
Skin rejuvenation is the focus of much of the experimental and clinical evidence for PBMT, and it is a popular option for treating redness, scars, acne, and wrinkles. The majority of clinical evidence is based on laser light (not LED) at the red and/or near-infrared end of the visible spectrum. However, there are a few well conducted studies using LED.
In a randomized, double-blinded, placebo-controlled, split-face clinical trial of LED phototherapy for skin rejuvenation, Lee et al observed enhanced fibroblast activity, collagen and elastin synthesis in response to LED light at 830 nm, 633 nm or both (alternately) for 20 minutes twice per week. Clinically, this manifested as reduced wrinkles and improved skin elasticity, which were measured objectively.
Most of the research showing positive results for repigmentation in dyschromias like vitiligo involves the use of laser therapy specifically.
Much of the data on the effect of PBMT on acne involves the use of blue light (sometimes in combination with red light) and the existing trial data are limited by short follow-up times, the exclusion of more severe acne in the study protocols, and a lack of direct comparisons with conventional acne treatments.
Noninvasive body contouring and/or localized subcutaneous fat reduction remains a hugely popular alternative to surgical fat loss strategies. LLLT is one of the existing independent, noninvasive modalities used in body contouring (along with cryolipolysis, high-intensity focused ultrasound, radiofrequency ablation). A number of proprietary laser-based LLLT devices, emitting light at the red/near-infrared end of the spectrum, have been investigated for their effect on the subcutaneous fat deposits of the abdomen, buttocks, thighs, and arms by way of clinical trials and case series. Despite industry funding and methodologic flaws, much of the data is favorable. That said, there remains a lack of evidence to support a role for LED-based LLLT in body contouring.
Interestingly, the mechanism by which LLLT reduces subcutaneous adipose deposits remains elusive. Hypotheses include light-induced transitory pore formation within lipid cell membranes with the loss of lipid contents into the interstitial space, alterations in adipocyte lipid metabolism, and/or a more systemic alteration in adipocyte behavior secondary to reduced oxidative stress with resultant increase in adiponectin secretion and reduction in insulin resistance.
Based on the most comprehensive review to date, “a reasonable body of clinical trial evidence exists to support the role of low-energy red/near-infrared light as a safe and effective method of skin rejuvenation, treatment of acne vulgaris and alopecia, and, especially, body contouring.”
Brain Health
Due to its effects on metabolic activity, inflammation, and blood flow, there has been increasing interest in the use of PBM for brain disorders, which can be classified into three broad groupings: traumatic events (stroke, traumatic brain injury, and global ischemia), degenerative diseases (dementia, Alzheimer's and Parkinson's), and psychiatric disorders (depression, anxiety, post traumatic stress disorder).
Non-invasive delivery of light from an external light source (laser or LED) to the head and then into the brain is commonly referred to as transcranial PBM. The majority of the research to date has been done in animal models, but some small clinical trials with humans do exist. The majority of the clinical investigations revealed positive impacts of transcranial PBM therapy in conditions such as TBI, stroke and depression, in which the target area was in the cortical regions of the brain.
In the early open studies in TBI, transcranial LED therapy (633/870 nm) improved self-awareness, self-regulation in social functioning and sleep quality.
The first study in patients with major depressive disorder showed that a single-session of LED therapy alleviated depression and anxiety symptoms (Hamilton scales) at 2 weeks post-irradiation. The use of transcranial high-power lasers ameliorated depression symptoms at 6–8 weeks post-irradiation in TBI cases with comorbid depression and in patients with depression alone. In the most rigorous clinical trial to date, Cassano and colleagues demonstrated a medium to large antidepressant effect size of near infrared light in patients with major depressive disorder.
Berman et al. applied PBM to elderly people diagnosed with dementia and noted an improvement in executive function and a significant improvement in sleep and mood.
Sexual Function?
The causes for sexual dysfunction are disparate and often multifactorial, including psychiatric, neurological, endocrine, cardiovascular and pelvic conditions, as well as side effects of commonly prescribed medications. The only rigorous, yet admittedly small clinical trial, to look at the effect of PBMT on sexual function used transcranial PBM to impact depression, which is often comorbid with sexual dysfunction. Despite directionally positive benefits, the study was exploratory in nature. There is no published data on the effectiveness of PBMT to improve erectile dysfunction in humans (only in rats with diabetes!). And there is no clinical evidence to substantiate widespread claims of increased testosterone with application of PBMT to the genitals.
Weight Loss?
Separate from its effect on body contouring, some studies suggest that there are benefits to red light therapy for weight loss. These studies use LLLT, not LED.
A recent 6-week pilot study in 60 people found that LLLT treatments twice per week led to a modest 0.8-inch (2-cm) reduction in waist circumference. A 2-week study in 86 people at a U.S. clinic observed a significant decrease in waist (1.1 inches or 2.8 cm), hip (0.8 inches or 2 cm), and thigh circumference (1.2 inches or 3 cm). Another study from 2017 also found that receiving LLLT in addition to treadmill walking resulted in a greater reduction in abdominal fat and body weight.
These studies are undermined by tiny sample sizes, inconsistent study designs, absent control groups, and short durations. No large-scale clinical trials have demonstrated that PBMT has a statistically or clinically meaningful weight loss benefit. Plus, there’s little data to show whether the existing results are long term or have clinical relevance. While some studies suggest that there are benefits to red light therapy for weight loss, claims from body sculpting/contouring companies often vastly overstate the effect.
Therapeutic Applications: Performance Effects of PBMT
Multiple studies show that PBMT can function as a performance-enhancing intervention in athletic activity and to enhance response and recovery to sports training regimens. In a comprehensive review of the effect of PBMT on sports performance and recovery, the authors report valuable protective and ergogenic effects of PBM from 25 human studies. PBMT before and/or after exercise seems effective for sports performance in both strength training and cardiovascular exercise training. This is presumably because it favors the reduction of muscle damage, decreases the concentration of lactate in the blood, increases the volume of oxygen and decreases muscle pain after exercise, all of which lead to an improvement in physical performance. However, there remains scarcity of scientific evidence on this subject needed to determine optimal wavelengths, the optimal times and whether therapy should be performed before or after exercise.
There is even the possibility that PBM could be used for cognitive enhancement in normal healthy people. Work by Gonzalez-Lima and his research team showed enhancement of higher-order cortical functions such as prefrontal rule-based learning, sustained attention, and short-term memory as well as executive functions in healthy young subjects.
PBMT: Risks–Benefits Analysis
Overall, the risks of PBMT are quite minimal. Red light and near infrared light wavelengths are nontoxic, noninvasive, and considered safe for all skin types. These wavelengths are not the same as ultraviolet (UV) light, which has been linked to skin cancer. Red light therapy can be easier to access than other treatments, as it can be done at medical or dental offices, spas, tanning salons, or beauty clinics. At-home devices are also widely available. It should be noted that for treating deep tissues, the wavelength of light used determines the depth of penetration into a tissue. In general near-infrared light penetrates more deeply than shorter wavelengths of light such as red light. Many new devices use mixed red and NIR wavelengths.
Currently, there are no set guidelines for how long or strongly red light therapy should be applied. While immediate results from red light therapy are possible, it is more likely going to take weeks or months to realize improvements. And the long-term safety of red light therapy is not known.
Final Take
Much of the preclinical and early clinical data for PBMT is quite promising. However the many controllable variables– wavelength, spatial coherence, polarity (the geometric orientation of the light wave with respect to the direction of travel), pulse structure, fluence (total energy), irradiance (energy density), and exposure frequency–have rendered PBMT challenging to study through clinical trials or systematic reviews. To date, the LED-based PBMT products marketed for skin rejuvenation, adiposity, joint pain, etc. use evidence largely extrapolated from preclinical data using laser devices. The clinical practice and commercialization of PBMT is far ahead of the clinical data.