Supplement Summary: NAD+
Definitions of the Major Players
NAD - Nicotinamide Adenine Dinucleotide, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups
NAM - Nicotinamide, a precursor to NAD
NR - Nicotinamide Riboside, a precursor to NAD
NMN- Nicotinamide Mononucleotide, a precursor to NAD
Niacin (Nicotinic Acid)- Vitamin B3, a precursor to NAD, NR, NAM, and NMN
Tryptophan - an amino acid, a precursor to NAD
NAD Consuming Enzymes - Sirtuins, PARPs, CD38
What is NAD and what does it do?
Currently, nicotinamide adenine dinucleotide (NAD) boosters are getting a lot of hype for their claimed anti-aging benefits. And, indeed, NAD+ was first discovered by regulating the metabolic rates of yeast extracts, and thus the links between NAD+ and metabolism have been known for almost a century. More recent evidence has demonstrated that NAD is a critical modulator of cell signaling and survival pathways in every single cell in the human body. Specifically, NAD allows cells to adapt to environmental changes including nutrient changes, DNA damage, circadian disruptions, infection, inflammation and toxicity from xenobiotics (chemicals that are foreign to human life). These effects of NAD are mainly achieved by the ability of NAD+ to transfer hydrogen in oxidation-reduction reactions. However, NAD is also an important cofactor for non-redox related reactions.
Cellular NAD exists in two forms, oxidized (NAD+) and reduced (NADH). During metabolism (i.e. glycolysis and fatty acid oxidation), NAD+ can be converted to NADH in the mitochondria (Krebs Cycle) by accepting an electron and this is called a cellular oxidation-reduction (redox) reaction. NADH is then responsible for carrying the hydrogen electron to the electron transport chain (ETC) to synthesize adenosine triphosphate (ATP). Regulation and maintaining a proper balance of the NAD+/NADH ratios is critical for normal cell function and viability. Both NAD+ and NADH play important roles as coenzymes in redox reactions in every single cell in the body, and an imbalance in their ratio can impair these pathways, resulting in dysregulated cellular metabolism.
In addition to redox reactions, multiple NAD+-dependent enzymes are involved in human physiology by chemical modification of DNA, RNA and/or proteins. Specifically, NAD+ is an essential cofactor for non-redox NAD+-dependent enzymes, including sirtuins (SIRTs), CD38 and poly(ADP-ribose) polymerases (PARPs). SIRTs are a class of signaling proteins that are involved in metabolic regulation and, potentially, aging. CD38 is a glycoprotein found on the surface of many immune cells and is important for the regulation of immune health and cancer prevention. PARPs are a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death.
Modalities of NAD+ Production and Therapy
NAD is able to be created in the body, naturally, using two dietary precursors - Niacin (Vitamin B3) and Tryptophan. However, because it is so critical to cell survival, NAD is also recycled from degradation in a so-called “salvage pathway”. There are three known pathways in which NAD is either created or recycled in the human body:
Priess Handler: Nicotinic Acid (NA), which is derived from vitamin B3, which can be obtained from foods such as meat, redfish, and nuts and is also produced by the microbiome, is converted into NAD+ through the Preiss-Handler pathway inside the cell.
De Novo Synthesis: This is the longest of the NAD+ synthesis pathways and is active mainly in the liver and kidneys. The amino acid tryptophan (Trp) is catabolized through the kynurenine (KYN) pathway (comprising 9 steps).
Salvage Pathway: This is the major pathway for NAD+ biosynthesis in most tissues in mammals. Enzymatic activities of NAD-consuming enzymes (SIRTS, PARPS, CD38) produce NAM as a by-product. NAM can also be obtained from the diet. In the salvage pathway, nicotinamide mononucleotide (NMN) is formed from NAM byproducts and then converted to NAD+. The cellular level of NAD+ can be increased either by NAD+ precursors NMN, NR, and NAM or by inhibition of enzymes that consume or degrade NAD+.
Source: Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021
NAD Homeostasis is critical for survival
It is important to note that NAD is not believed to enter the cell from the bloodstream. Instead, it must be converted to NR (or potentially NMN) in order to be absorbed by the cell. Once inside the cell, NAD+ is highly compartmentalized in the cytoplasm, mitochondria and nucleus, which represent its main intracellular pools. These pools are regulated independently of each other, and consistent with this, the enzymes involved in the biosynthesis or degradation of NAD+ are highly compartmentalized as well.
The intracellular concentration of NAD+ is a balance between NAD+ consumption and synthesis. NAD+ is also continually turned over by three classes of NAD+-consuming enzymes: SIRTs, PARPs, and CD38 (among others). These utilize NAD+ as a substrate or cofactor and generate nicotinamide (NAM) as a by-product. To sustain NAD+ levels, NAM can be recycled back to NAD+ via the NAM salvage pathway. Additionally, some cells, mostly in the liver, can synthesize NAD+ de novo from multiple dietary sources. Thus, NAD+ is constantly synthesized, catabolized and recycled in the cell to maintain stable intracellular NAD+ levels.
Depletion of NAD+ Affects Cellular and Systemic Health
Although medical doctors and scientists were unaware of the implications of NAD depletion in the early 1900’s they recognized a disease they came to call “pellagra”, whose symptoms include the 3 D’s: dermatitis, diarrhea and dementia. If untreated, pellagra can lead to a 4th D: death. They categorized this disease as a systemic disease caused by the severe deficiency of Niacin (Vitamin B3). Pellagra, it is now known, is the dramatic manifestation of a severe and chronic NAD+ depletion.
While pellagra is no longer a major health concern, reduced levels of NAD+ coupled with a shift toward NADH are now considered a hallmark of aging, although the underlying causes remain unclear. During aging, NAD+ levels decline by between 10% to 65% overall, with many enzymes associated with NAD+ degradation and biosynthesis also altered. This decline in NAD+ levels during aging has been linked to the development and progression of aging-related diseases, including atherosclerosis, arthritis, hypertension, cognitive decline, diabetes and cancer. Some potential causes of NAD depletion are mitochondrial dysfunction and changes in the volume, integrity, and functionality of mitochondrial DNA, increased reactive oxygen species accumulation and chronic inflammation.
Independent of aging, NAD depletion has also been shown to occur with the consumption of a high fat diet, post-partum weight loss, lack of sleep, and high levels of alcohol consumption. However, because NAD is extremely challenging to measure in humans due to its metabolic volatility, these studies have only been examined in vitro (cell) and animal models.
Source: Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020
Physiological Response to NAD+ Supplementation/Infusion
NAD+ and its metabolites systemically flux and exchange across tissues, with a tissue-specific distribution of NAD+ biosynthetic enzymes and a tissue-specific preference for specific NAD+ precursors. There are several supplementation methods that can be utilized to increase NAD+ including: Niacin, Tryptophan, NR, NMN and NAM oral supplementation. Each have been implicated in the subsequent biosynthesis of NAD+. To date, there are very few human studies examining IV or IM applications of NR, NMN, or NAD in humans. As a result, the mechanisms by which these therapies alter human physiology is largely unknown.
Niacin and Tryptophan: It is reported that the de novo biosynthesis of NAD+ from tryptophan mainly occurs in the liver and, to a lesser extent, kidney, which is attributed to the exclusively expressed enzymes involved in de novo NAD+ synthesis in these tissues. Niacin metabolite, NA, can be directly transported into the cell for utilization. Therefore, the concentration of niacin and tryptophan in the diet affects the liver NAD+ levels. Tryptophan also compensates the NAD+ biosynthesis when the salvage pathway is blocked.
NMN and NR: Most studies on NMN and NR are preclinical studies in rodents. Outcomes from these trials suggest a strong translational potential for NAD+-boosting therapies. However, studies in humans are less advanced, and to date the clinical trials evaluating the pharmacokinetics and toxicology of oral NAD+ and its precursors have primarily demonstrated that oral administration is safe and can efficiently increase NAD+ levels in healthy volunteers. Of note, there have been more phase I clinical trials using NR than those using NMN, and these have provided conflicting results. It should be noted that the use of NMN as an oral supplement has recently been disallowed by the FDA.
Injectable NMN, NR, and NAD: Several studies have been conducted in rodents examining intraperitoneal, subcutaneous, and intravenous applications of NMN and NR over several days through approximately one year. There are limited studies in humans examining injectable NMN and NR. To date, only one study has examined injectable NAD in humans. The study was conducted to understand whether NAD+ infusion increased NAD+ and it’s metabolites in human plasma. Results concluded that over 6-hours of infusion, NAD+ was rapidly metabolized by cells in the first two hours of administration but not after. The researchers hypothesized that enzymatic saturation impaired cellular metabolism and uptake after 2 hours, and the remainder was mostly cleared through urine. No physiologic outcomes or measures of cellular uptake/utilization were reported.
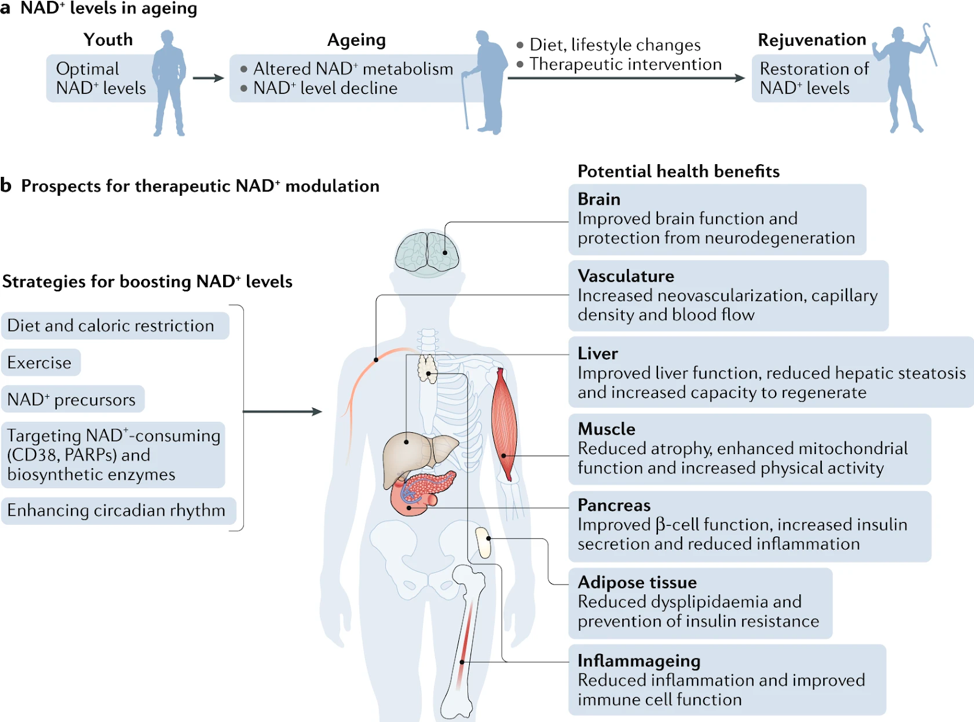
Health Impact of Increasing NAD+
There are several strategies known to boost NAD+ levels including lifestyle changes, such as exercise, time restricted eating, eating a healthy diet and following a consistent daily circadian rhythm pattern (i.e. sleep and meal consistency). Another approach is the use of oral or injectible NAD+ precursors, such as NMN, NR, and NAD+. All of these approaches promote tissue NAD+ levels and are beneficial for health. These include improved tissue and organ function, protection from cognitive decline, improved metabolic health, reduced inflammation and increased physiological benefits across cell, rodent and human models, which may collectively extend healthspan and potentially lifespan. Scientists are also examining strategies to modulate NAD+ biosynthetic enzymes, in particular those that regulate the rate-limiting step of de novo synthesis and salvage pathways, and the inhibition of enzymes involved in NAD+ degradation, such as PARPs and CD38.
Source: Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021
Preclinical studies in aging models show that increasing NAD+ levels reduces age-related immune and metabolic changes and could potentially be used as a therapeutic strategy for treating aging-associated pathologies. The role of NAD+ in various diseases has been evaluated using genetically modified mice or by boosting or replenishing NAD+ levels by administering precursors of NAD+ biosynthesis. In the sections below, we will focus only on summaries of outcomes in human studies, some of which conflict with preclinical data. Comprehensive reviews of human data can be found here, here, and here.
Metabolic and Cardiovascular Disorders
To date, very few human studies have been conducted that examine metabolic and cardiovascular health outcomes. Populations included, and subsequent effects have been mixed. When taking an oral NMN supplement, women with prediabetes were shown to have improved insulin sensitivity. However, when examining NR oral supplementation after 12 weeks, no effect on insulin sensitivity or glucose metabolism was noted in patients with prediabetes that also had overweight or obesity. In those with hypertension, NR decreased blood pressure and arterial stiffness in healthy middle aged and older adults and decreased proinflammatory cytokines in patients with heart failure.
Liver Health
When investigating liver health, researchers noted NR, in combination with glutathione precursors and L-carnitine improved liver enzymes (ALT/AST) in patients with NAFLD. Additionally, in a small study, improvements in hepatic lipid content was observed when individuals with obesity and prediabetes were supplemented with oral NR.
Aging
While many parameters of longevity and aging related to increased in NAD+ have been noted in animal models, only a handful have examined humans. NR has been shown to improve muscle mitochondrial function in older adults along with improved exercise performance. Additionally, researchers have noted reduced systemic inflammation after oral NR in the elderly. Finally, after 12-weeks of NMN oral supplementation older adults experience improved sleep quality, fatigue and physical performance.
Brain Health
One study in humans, called NADPARK, demonstrated increased brain NAD levels, transcription of mitochondrial genes and decreased inflammatory cytokines in patients with Parkinson's disease after supplementation with NR.
Performance Effects
With regard to physical performance, there are several studies that have been conducted with varying outcomes. Interestingly, it has been demonstrated that NR, using a specific transporter, is preferentially used by muscle to synthesize NAD+. One study examined NMN oral therapy in healthy amateur runners and found enhanced aerobic capacity, likely the result of enhanced O2 utilization of the skeletal muscle. However, in two conflicting studies, examining oral supplementation of NR, no effect was noted on muscle performance or response to exercise, nor on muscle mitochondrial content or respiratory capacity.
Registered Trials
There are several clinical trials registered for enrollment and completion over the next several years that include trials in hypertensive individuals, people with polycystic ovary syndrome and diminished ovarian reserve, as well as improvements in physical activity.
Safety, Efficacy, and Effectiveness Considerations of to NAD+ Therapies
Early-phase trials of short-term NR/NMN administration, as well as IV NAD+ have demonstrate the short-term safety and effective increase in NAD+ levels in healthy participants. Of note, there may be even improvements in health outcomes when NAD+ and its precursors are combined with other compounds that exhibit a protective health effect. Perhaps the maximum benefit can be derived not simply from one compound but from an ingredient profile that incorporates multiple compounds that work together to achieve a synergistic effect.
However, despite the promising preliminary results, whether long-term supplementation with NAD+ precursors has any side effects is still unknown. One study of supraphysiologic doses of NR in a tumor generative mouse model (comparable to a human ingesting 30 grams of NR per day) induced greater tumorigenesis in the brain compared to control. While these data are important to acknowledge, there is likely an upper limit toxicity effect that needs to be determined both in rodents and humans.
Overall, more data is critical to understand human physiology with NAD+ application, and its precursors, health outcomes, dosage and safety.